In struggle over mifepristone, generic abortion capsule maker sues FDA : NPR
[ad_1]
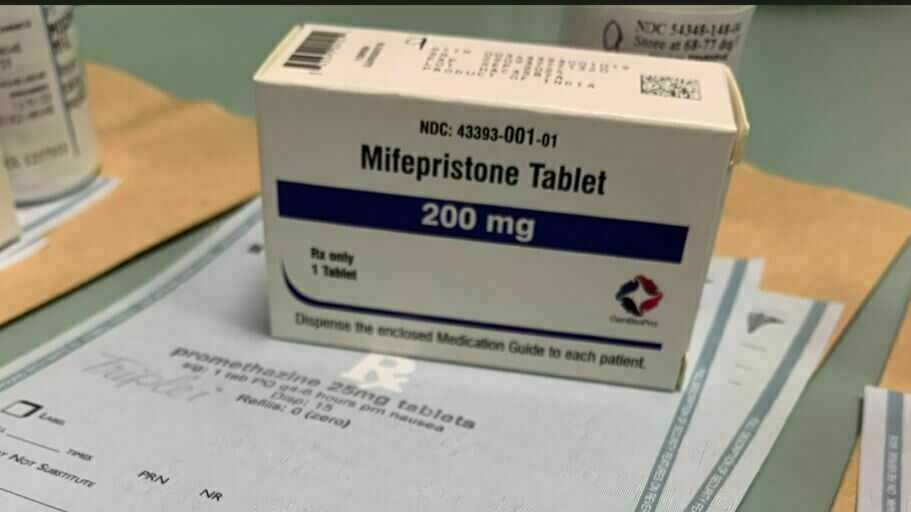
The drug producer GenBioPro acquired FDA approval for its generic model of the abortion capsule mifepristone — the primary dose in a widely-used, two-drug protocol — in 2019.
Sarah McCammon/NPR
disguise caption
toggle caption
Sarah McCammon/NPR
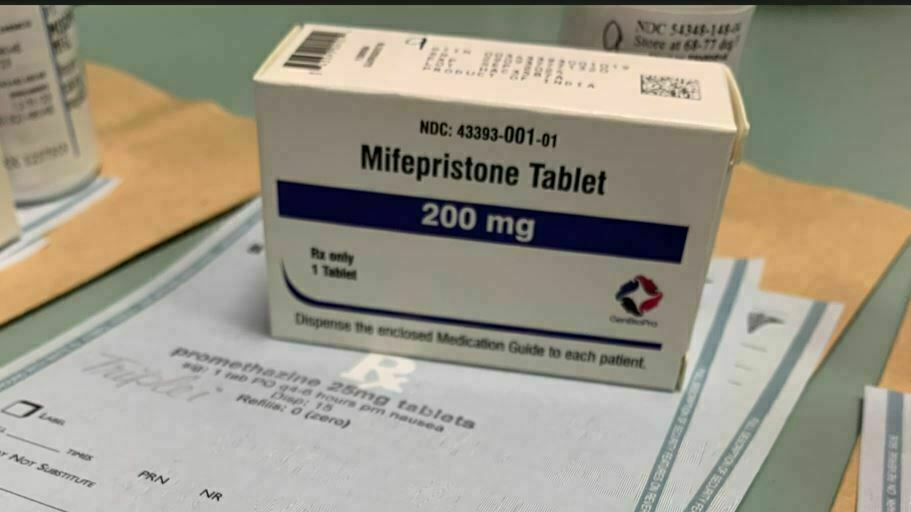
The drug producer GenBioPro acquired FDA approval for its generic model of the abortion capsule mifepristone — the primary dose in a widely-used, two-drug protocol — in 2019.
Sarah McCammon/NPR
The producer of a generic type of the abortion capsule mifepristone is suing the Meals and Drug Administration in an effort to protect entry as federal litigation threatens to overturn the FDA’s approval of the drug.
In a federal lawsuit filed right now in Maryland, drug producer GenBioPro asks a decide to ban the FDA from taking any motion that may disrupt entry to the drugs. GenBioPro says revoking the FDA approval of generic mifepristone would trigger “catastrophic hurt” to the corporate, and to docs and sufferers who depend on the drug.
Mifepristone was first authorized in 2000 as the primary dose in a widely-used, two-drug protocol authorized to induce some first trimester abortions. GenBioPro acquired FDA approval for its generic model in 2019.
Anti-abortion rights teams are difficult each the FDA’s unique 2000 determination and later rule modifications, together with the generic drug approval in 2019.
A short-term keep from the U.S. Supreme Courtroom preserving status-quo entry to mifepristone expires at 11:59 p.m. ET right now except the court docket intervenes. If the keep expires, an order from the fifth U.S. Circuit Courtroom of Appeals would take impact and impose a number of restrictions, together with prohibiting the drugs from being distributed by mail.
In a submitting with the Supreme Courtroom, the FDA says it additionally believes that underneath the Fifth Circuit determination, generic mifepristone “would stop to be authorized altogether.”
Within the new lawsuit, GenBioPro objects to the FDA’s interpretation of that call and asks a federal court docket to drive the FDA to protect entry. The corporate says its generic type of the drug accounts for about two-thirds of mifepristone offered in the USA.
In a press release, Skye Perryman with the authorized advocacy group Democracy Ahead Basis and one of many attorneys within the case, stated the end result might have bigger significance for different medicines.
“There are business huge implications if far-right exterior curiosity teams
are capable of intervene with drug availability within the nation with out the authorized and regulatory protections supplied by Congress,” Perryman stated. “If this had been to be the case, few corporations could be incentivized to develop and produce important medicines to market.”
Danco Laboratories, the unique distributor of mifepristone within the U.S., has joined the FDA within the case and is asking the Supreme Courtroom to dam restrictions on the drug.
In a separate case filed earlier this 12 months, GenBioPro additionally sued the state of West Virginia over its state abortion restrictions, arguing that federal laws permitting using mifepristone ought to prevail over West Virginia’s state legal guidelines.
[ad_2]


No Comment! Be the first one.