New remedy to stop critical RSV signs in infants could also be accepted quickly : Photographs
[ad_1]
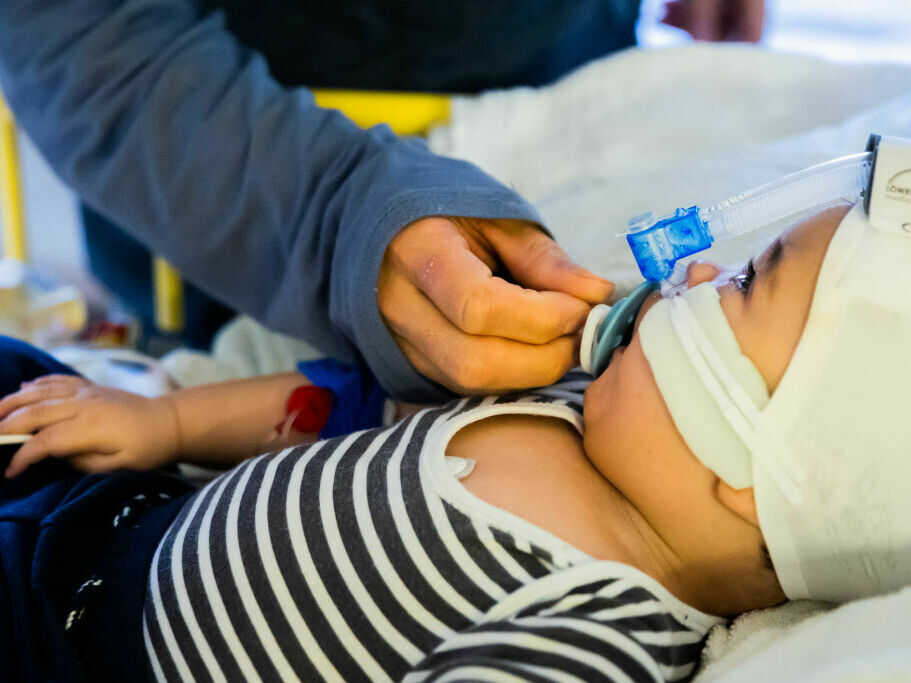
Every year, RSV infections ship as much as 80,000 children beneath 5 to the hospital for emergency remedy. A brand new antibody remedy might shield the youngest children — newborns and up infants as much as 2 years outdated.
Christoph Soeder/dpa/image alliance through Getty I
conceal caption
toggle caption
Christoph Soeder/dpa/image alliance through Getty I
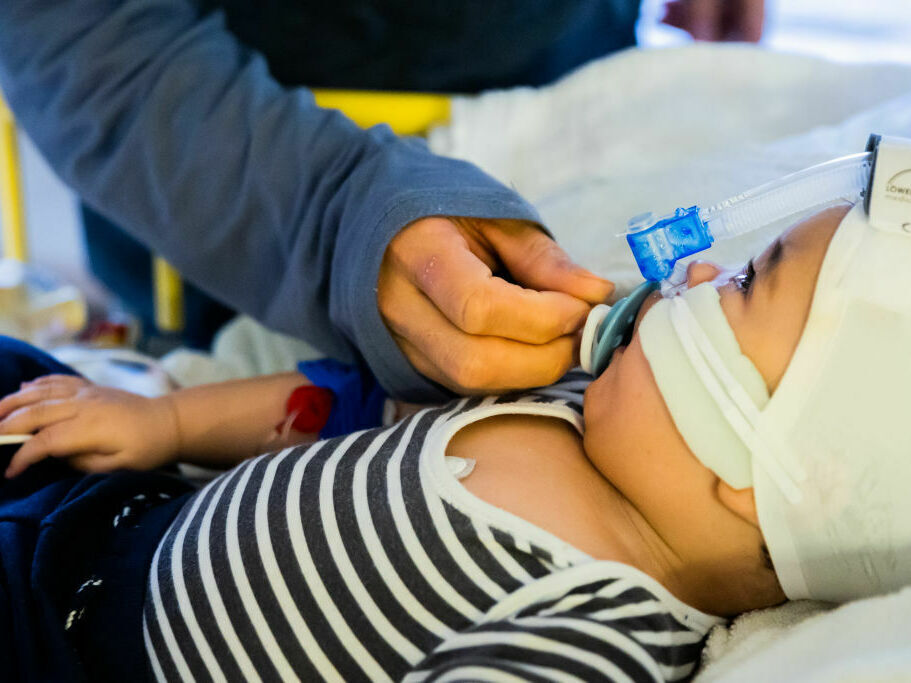
Every year, RSV infections ship as much as 80,000 children beneath 5 to the hospital for emergency remedy. A brand new antibody remedy might shield the youngest children — newborns and up infants as much as 2 years outdated.
Christoph Soeder/dpa/image alliance through Getty I
Cheryl Meany, a highschool instructor from Camillus, N.Y., was excited when she discovered she was carrying twins in 2014. However her pleasure shortly turned to fret as docs flagged a number of well being issues, together with potential mind lesions.
So she wanted a second to course of when her husband, a respiratory therapist, proposed enrolling the soon-to-be-born infants in an experimental examine for an unrelated sickness. It was a trial for a protecting remedy for RSV or respiratory syncytial virus, a typical respiratory virus that may be fairly extreme in younger kids.
“It took me aback, like ‘What are you even speaking about? I do not even know what you are asking me proper now,'” Meany stated.
That was in 2014, a number of years earlier than the current RSV surge overwhelmed hospitals throughout the nation. However Meany was fearful concerning the sickness again then after seeing a few of her mates’ children find yourself within the hospital from it. As much as 80,000 kids beneath 5 are admitted for RSV every year.
So she enrolled her daughters within the trial for a monoclonal antibody that works to stop RSV-induced decrease respiratory tract an infection in infants. Her resolution helped transfer ahead one of the vital promising remedies to guard infants from extreme impacts of RSV in many years.
In January, drugmakers AstraZeneca and Sanofi introduced the U.S. Meals and Drug Administration is formally reviewing their utility to get the remedy – known as nirsevimab – accepted within the U.S., together with outcomes from the trial the Meany twins joined.
AstraZeneca stated its third part trial outcomes confirmed its single-dose remedy was almost 75% efficient at stopping extreme an infection in infants all through an RSV season. The information was printed in March 2022 within the New England Journal of Drugs.

Earlier than they have been born in 2015, twins, Stella and Cassidy Meany (left to proper), have been enrolled in a trial for a preventative remedy for RSV. The remedy could quickly be obtainable to guard newborns in opposition to the respiratory sickness.
Cheryl Meany
conceal caption
toggle caption
Cheryl Meany

Earlier than they have been born in 2015, twins, Stella and Cassidy Meany (left to proper), have been enrolled in a trial for a preventative remedy for RSV. The remedy could quickly be obtainable to guard newborns in opposition to the respiratory sickness.
Cheryl Meany
Dr. William Schaffner, medical director on the Nationwide Basis for Infectious Ailments who was not concerned on this analysis, stated the outcomes counsel nirsevimab might considerably scale back the numbers of infants which might be hospitalized every year for RSV.
“The potential influence in assuring a wholesome infancy for a really massive proportion of the infants born right here in america — and even past — is probably very, very massive,” Schaffner stated.
A type of ‘passive immunization’
The drug – a long-lasting antibody injection – is meant for newborns or different infants dealing with their first RSV season, and for infants as much as 24 months of age of their second RSV season, in keeping with AstraZeneca’s press launch.
Dr. Joseph Domachowske, a pediatric infectious illness specialist at Upstate Medical College Hospital in Syracuse, helped launch the earliest part of the nirsevimab examine.
“RSV is the primary motive why infants and younger kids are hospitalized, not simply within the U.S., however internationally,” he stated.
He defined that the antibody remedy shouldn’t be a vaccine however is what scientists seek advice from as “passive immunization.” The antibodies in opposition to RSV flow into within the infants’ our bodies defending in opposition to the virus, ought to the kid be uncovered.
“It would not trigger an immune response itself,” he stated, and it additionally would not trigger the physique to develop immune reminiscence. “Nevertheless it offers safety for a time frame till it wears off,” he stated. An identical kind of remedy was used to assist shield immunocompromised sufferers in opposition to COVID.
Domachowske, who additionally led the hospital’s COVID-19 vaccine trial for teenagers, expects a greenlight from regulators in time to have nirsevimab obtainable by the following RSV season within the fall. It has already been accepted in Europe.
Lengthy journey to an efficient remedy
When Meany’s daughters acquired their injections in January 2015, they have been the primary infants on the earth to obtain it, in keeping with AstraZeneca.
Domachowske, a Meany household pal, stated giving the dual infants safety in opposition to RSV was a big second after researchers had struggled for years to discover a remedy to stop RSV. Again within the Nineteen Sixties, a totally different remedy, a vaccine candidate, was beneath examine. Nevertheless it made children sicker from RSV – and two infants died from it.
“It actually charged up the flawed half of the immune system,” Domachowske stated.
Progress did not come till twenty years later. In 1998, the FDA OK’d a monoclonal antibody for untimely and high-risk infants. However Domachowske stated altering medical pointers since then have severely restricted eligibility for this remedy, and, he stated, its efficacy wasn’t nice.
“It needs to be given month-to-month,” Domachowske stated. “And it is efficient at stopping hospitalization, not efficient at stopping an infection.”
That is the place the analysis had been caught for years till 2014, when Domachowske attended a medical convention in Argentina. A featured speaker dropped an enormous discovery that a variety of RSV analysis targeted on the flawed protein.
“Everyone seems to be sitting there staring with their mouths gaping open like, ‘For this reason all of our work hasn’t led to something for many years,” Domachowske stated. “It was that spectacular. And you may see the pharma folks that have been attending, taking notes, calling their colleagues saying, ‘Cease, cease the work.'”
Not too lengthy later, he injected Meany’s daughters with an improved, longer-lasting monoclonal antibody that protects infants via an RSV season with one shot.

The Meany twins have been the primary on the earth to get photographs of nirsevimab throughout early trials once they have been infants. That they had no negative effects and no signs of RSV.
Cheryl Meany
conceal caption
toggle caption
Cheryl Meany

The Meany twins have been the primary on the earth to get photographs of nirsevimab throughout early trials once they have been infants. That they had no negative effects and no signs of RSV.
Cheryl Meany
The dual women, Cassidy and Stella, are actually 8 years outdated and wish to compete in ninja warrior contests — they race via impediment programs that characteristic ladders, monkey bars and overturned Bosu balls.
Meany stated the women by no means had issues from the shot and by no means displayed signs of RSV. She is happy with the function they performed in medical historical past.
“This issues, and this issues for teenagers in every single place, not simply children right here,'” Meany stated.
Domachowske stated the women could have gotten RSV in later seasons after the results of the remedy had worn off. However since older kids’s immune techniques are stronger, signs weren’t noticeable.
A welcome RSV prevention software
Physicians and infectious illness specialists welcome the potential approval of the remedy.
Schaffner of the Nationwide Basis for Infectious Ailments stated if it have been already accepted within the U.S., nirsevimab would’ve helped curb the excessive charge of infections seen this season, one of many worst current seasons for the illness.
“This current surge would have been remarkably blunted,” he stated.
Dr. Vandana Madhavan, scientific director of pediatric infectious illness at Mass Basic for Kids stated the monoclonal antibody is a big achievement within the battle in opposition to RSV.
“This can be a large step ahead,” she stated.
[ad_2]
No Comment! Be the first one.